The Effect Of Thermal Massage On Human T-Lymphocyte And Natural Killer Cell Function
C So, J Sarath, R Giolli, S Gollapudi
Citation
C So, J Sarath, R Giolli, S Gollapudi. The Effect Of Thermal Massage On Human T-Lymphocyte And Natural Killer Cell Function. The Internet Journal of Alternative Medicine. 2007 Volume 6 Number 1.
Abstract
The purpose of this pilot study was to assess the effect of thermomechanical massage on human immune cell function. Immunological studies were performed on 10 healthy volunteers (average age: 24 years). Subjects were asked to lie on a thermomechanical massage device (TMD), for 20 minutes, twice a week, for a total of 8 weeks. Blood samples were collected before entry into the study and at the beginning of the 16th session. Peripheral blood mononuclear cells were isolated and were evaluated for immunophenotypic characteristics as well as for the functional parameters of T-lymphocytes (T cells) and natural killer (NK) cells. T cell proliferation was assessed by 3H-thymidine incorporation assay. NK cell mediated cytotoxicity was determined by a non-radioactive cytotoxicity assay kit using flow cytometry. Immunophenotyping of the peripheral blood lymphocytes showed increased percentages of T cells (CD3+), T cell subsets (CD4+ and CD8+), and NK cells (CD16+CD56+). Functional analysis revealed a significant increase in T cell proliferative response to PHA, a polyclonal T cell mitogen, and a significant increase in NK cell cytotoxicity following the use of TMD. We conclude that use of TMD twice a week may modulate immune function and may be beneficial in subjects with impaired immune function, such as aging individuals and HIV patients.
Introduction
A considerable number of people in the United States and across the world use “complementary and alternative medicine” (CAM) for prevention and treatment of different health ailments (1,2,3). In 1997, visits to chiropractors, massage therapists, and acupuncturists combined represented 55% of all visits to CAM practitioners (6). Surveys of the U.S. population indicate that 3% to 16% of adults receive chiropractic manipulation, while between 2% and 14% receive massage therapy (1, 4,5) . People reported using these therapies for a variety of conditions, including relief of stress, pain, neurological problems, and inflammatory conditions(7).
Stimulation of acupuncture points either through massage, insertion of needles or via heat (moxibustion) are shown to induce many physiological effects, e.g. relaxation of skeletal muscle (8,9) increase in peripheral blood lymphocytes and lymph circulation (10,11,12,13), reduction of anxiety (14,15), and activation of the autonomic nervous system (16,17,18,19). In addition, an alteration in the number and function of lymphocytes, NK cells, and phagocytic cells have been reported post-treatment with acupuncture (20, 21) and therapeutic massage (22–23). Accumulating evidence suggests that the autonomic nervous system is involved in regulating the levels of immune cells within the circulation, their activity, and the subsequent magnitude of a cellular response (24, 25). Other studies using new imaging modalities, such as functional MRI and PET scans, have revealed a direct effect of acupuncture points on the function of different brain centers (26, 27).
During the last few years, the thermomechanical massage device (TMD) was introduced to the US market. This device combines the principles of acupressure, moxibustion, and manipulation (Shiatsu of Japan, Chuna of Korea and chiropractic). Preliminary studies indicate that musculoskeletal, gastrointestinal and nervous system problems were the most common conditions for which TMD was used and improvements were reported in 91% of the cases (28). In another study, a statistically significant drop in systolic and diastolic blood pressure were noted in 35 hypertensive subjects following use of TMD, and a similar drop in fasting and 2hour post-prandial blood sugar were also reported (7)
A new generation of TMD provides electronic heating with Helium-Jade stones which provides for a continual massage of muscle groups to enhance the blood circulation. Another new addition to the device is the infrared rays that penetrate the surface of the body so that the mechanical massage probes stimulate the autonomic nervous system and dilate capillary blood vessels of the neck, thorax, lumbar spine, and sacral region. Furthermore, the TMD also has an external 15-way jade massage probe that stimulates the surface of the abdomen and pelvis, resulting in increased blood flow to those areas. Currently, little is known about the effect of TMD on the function of the immune system. In the present pilot study, we examined the effect of using TMD on the immune system of healthy young adults.
Methods
This pilot study is a pre-post study design, with blood samples to assess changes in cellular immunity following participants’ use of TMD. The study was approved by the Institutional Review Board at the University of California, Irvine (UCI).The TMD used in this study is an FDA approved mechanical massage bed (Migun Hy 7000, Migun Company, Los Angeles, CA). TMDs are known to apply both heat and mechanical massage to selected portions of a user’s anatomy. Migun 7000 provides full thermal massage treatment from head to toe. The thermal acupressure bed also is a far infrared heating device and the infrared lamps included in the product provide topical heating. A detailed description of the device and its design are given at the manufacturer’s website (Migun.com). Briefly, the HY 7000 bed consists of an internal projector with heated jade rollers and external 15 way jade massage heads. The thermal massage treatment uses helium to produce heat and uses jade massage heads to create pressure. The internal heated massage heads move slowly along the spine and contour of the body from neck to the sacral region and from the posterior compartment of the ankle to the hips. The external heated massage heads are designed to deliver the infrared heat to other areas of the body. According to the manufacturers, the device was designed based on acupuncture theory originating in China more than 2,500 years ago.
Ten healthy adult volunteers were recruited from the general UCI population through local advertisement. Five male and five female volunteers, average age of 24 years, met the inclusion criteria. Subjects were asked to participate in 2 sessions each week for 2 months (total of 16 sessions). Each session started at 8:30 A.M. and the subjects rested for about 30 minutes and at 9 A.M they started the massage session . The massage session consisted of lying on the TMD for approximately 20 minutes. Thermal massage was administered as per the instruction of manufacturer.
Vital signs, weight, and a brief medical history were obtained prior to each session by the General Clinical Research Center (GCRC, University of California, Irvine, CA) nursing staff at UCI. Blood samples were collected from each participant twice, prior to the first session and at the completion of the study (2 months). . Blood samples collected at 2 months were used to evaluate the possible effect of the TMD on the immune system and results were compared with the initial blood sample (time 0) that was used as a baseline control. Because leukocytes have circadian rhythms (reference), we obtained blood samples at the same time from each donor. Both the initial and post treatment blood samples were drawn prior to massage session i.e. 9 AM.
Blood samples were analyzed to evaluate the proportions and absolute number of T cell subsets (CD3+, CD4+ and CD8+), NK (CD16+ CD56+) cells, and T cell and NK cell functions. All tests were performed using freshly drawn blood. The following tests were used for this study:
The percentage of lymphocytes and absolute number of cells determined by flow cytometry: A direct two-color flow cytometry was performed on whole peripheral blood using the following reagents: fluorescein (FITC)-conjugated pan T cell marker CD3, phycoerythrin (PE)-conjugated anti-CD4 (Helper T cell marker),anti-CD8 (cytotoxic T cell marker), or anti-CD16+ 56+ (NK cell marker), and FITC-mIgG with PE-mIgG (isotypic controls) (all reagents from Becton- Dickinson Biosciences, San Jose CA). In addition, a cocktail combination of antibodies directed to CD4 (PE) or CD8 (PE), and FITC CD69 or CD25 (activation markers) were used. Antibodies (20 µl) were incubated with 100 µl of whole blood according to manufacturer’s instructions. The red cells were lysed and the lymphocytes were fixed with 1% paraformaldehyde solution. Flow cytometry analysis was performed using FACScan. Single and dual color analyses were evaluated on lymphocytes bit-mapped by forward and side scatter. Data were analyzed with Simulset Software (Becton Dickenson, San Jose, CA). The percentage of lymphocytes and the absolute numbers of cells were determined.
Isolation of lymphocytes: For functional analysis, peripheral blood mononuclear cells (MNCs) were isolated by Ficoll-Hypaque density gradient centrifugation of blood from study participants. MNCs were washed twice with Hanks Balance salt solution (HBSS)and re-suspended in serum-free AIM V medium (GIBCO, Long Island, NY).
Determination of T cell function: In this study we examined the effect of TMD on the proliferative response of T cells to the polyclonal mitogen phytohemagglutinin (PHA, a T cell mitogen) by the 3 H-thymidine incorporation assay. Briefly, MNCs (2 x 10 5 /well) were cultured in triplicate, in round bottom tissue culture plates at 37 0 C, in the presence or absence of PHA (10 µg/ml), for 3 days. Twenty-four hours prior to termination of culture, 1 µCi of 3 H-thymidine was added to each well. Cultures were harvested and 3 H-thymidine incorporation into DNA was determined by a scintillation spectrometer.
Determination of NK cell function: NK cell mediated cytotoxicity was determined by a non-radioactive cytotoxicity assay kit (ACT1, Cell Technology Inc., Mountain View, CA), using flow cytometry according to the manufacturer’s instructions. Briefly, human erythroleukemic tumor cells, K562, were used as target cells (1 x 10 4 cells) were labeled with the cell tracking dye CFSE (laser emission, FL1) and were then cultured with peripheral blood mononuclear cells (2.5 x 10 5 cells) at an effector:target ratio of 1:25. After a 6 hour incubation at 37°C, live dead stain 7AAD or propidium iodide (PI, Laser emission FL3 channel) was added to measure cell death. 7AAD and PI only enter membrane-compromised cells and bind to DNA and thus stain dead cells. For each sample, data from 10,000 cells were collected by FACScan flow cytometer and analyzed. During analysis an electronic gate was placed on CFSE-labeled target cells and dead (7AAD or PI positive cells) were determined.
Statistical Analysis: Data are reported as means ± S.D. Mean differences between pre- and post-treatment values were analyzed with paired t test. Values of P < 0.05 (two-tailed) were considered significant.
Results
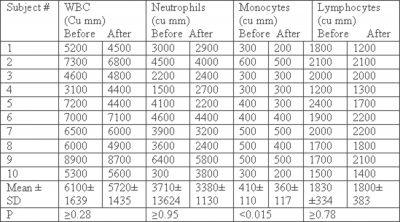
White blood cell (WBC) count: Measurement of WBC count was performed before the beginning of the study and at 2 months. Table 1 represents a compilation of the mean values for several hematological parameters of the peripheral blood from the study subjects before and after TMD therapy. Comparisons of pre- to post-peripheral WBC count measures revealed no significant change in the total number of WBCs or neutrophil counts. However, there was a small, insignificant decrease in lymphocyte counts. On the other hand, monocyte counts were decreased significantly post massage treatment (P< 0.015).
Figure 1
Table 1: Leukocyte and differential counts Before and after Migun Massage Device
Peripheral immunophenotype of T-lymphocyte subpopulations:
Cytofluorographic analysis for the evaluation of T cell subset populations revealed that TMD therapy significantly increased the proportion of total T cells (CD3+ cells), and T cell subsets, CD4+ and CD8+ T cells (Figure 1). However, TMD treatment caused an insignificant difference in the absolute numbers of CD3+, CD4+ and CD8+ T cells (Figure 2)and the CD4:CD8 ratio (1.34 ± 0.45 vs. 1.25 ± 0.46, P>0.07).
Figure 2
Figure 1: Change in Peripheral Relative Proportions of T-Lymphocytes, after TMD in Normal Subjects. Peripheral blood was stained with FITC labeled anti-CD3 and PE labeled anti-CD4 or CD8 and the proportion of T cell subsets was determined by dual color flow cytometry. The results represent mean ± SD, N = 10. Asterisks indicate significant changes relative to the pre TMD (before) values(paired Student’ test): CD3 *P<0.003, CD4 *P<0.029 and CD8 **P<0.006.

Figure 3
Figure 2: Changes in absolute number of Peripheral CD3+ (total T cells) CD4+ (Helper) and CD8+ (cytotoxic T cells) and CD4:CD8 Ratio, after TMD. Data represent mean ± SD, N = 10. The observed changes are not statistically significant ( paired student t test). P values are, CD3 P?0.26., CD4 P ?0.3., CD8 P?0.08., CD4/CD8 p ?0.07.

Peripheral T cell function
In order to evaluate the effect of TMD on T cell function, we tested T cell proliferation in a classical mitogen assay employing the T cell-specific mitogen PHA (Figure 3). There was a statistically significant increase (P≤0.04) in the proliferative response of lymphocytes derived from the subjects subsequent to massage as compared to their baseline values.
Figure 4
Figure 3: Effect of TMD on T cell function: Peripheral blood mononuclear cells were stimulated with PHA (10µg/ml). Proliferation was determined by measuring H thymidine incorporation into DNA. Data are shown with mean ± SD, N = 9. *p < 0.04 as compared with proliferative response of MNC obtained prior to massage ( paired student t test).
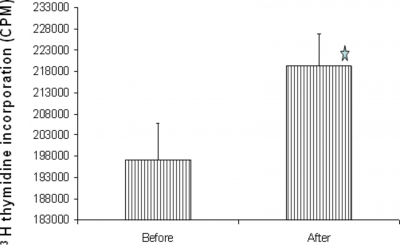
Figure 5
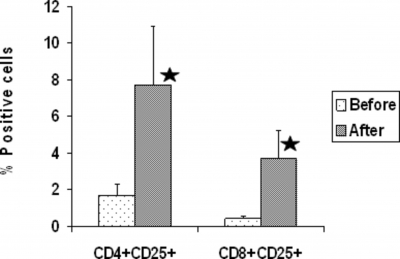
Figure 4: Effect of TMD on IL-2 receptor expression on CD4+ and CD8+ T cells. IL-2 receptor expression was determined by staining the cells with FITC labeled antibody to IL-2 receptor and PE labeled anti- CD4 or CD8 antibody and FACscan flow cytometer. Data are shown with mean ± SD. N= 10. Asterisks indicate significant changes relative to the pre TMD (before) values(paired Student’ test): CD4 *P<0.023, and CD8 **P<0.025.
NK cell number and Function: The proportion and absolute number of NK cells and NK cell function was determined by flow cytometry (Figure 5).
Figure 6
Figure 5: Changes in Relative Proportions, number and Functions of NK cells, after TMD in Normal Subjects. Peripheral blood was stained with FITC labeled anti-CD3 and PE labeled anti-CD16 and CD56 and the proportion of NK cells (CD16+CD56+) cells was determined by dual color flow cytometry. NK cell function was determined by incubating Peripheral blood lymphocytes (effector cells) with K562 (Target) cells at an effector target ratio of 25:1 for 4 hr. Target cell death was determined by FACScan flow cytometer. Asterisks indicate significant changes relative to the pre TMD (before) values (paired Student’ test): % NK ( CD16+ CD56+) *P<0.046, % Cytotoxicity **P<0.0036.
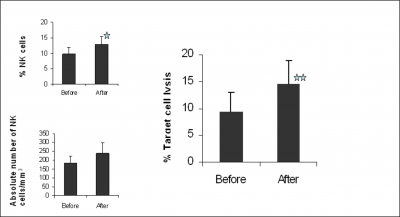
TMD treatment resulted in significantly increased relative proportions of CD16+ CD56+ NK cells from 8.3 ± 4.9% to 13.6 ± 7.9% (P = 0.05). Therapy also increased the absolute number of NK cells from 150.5 (±94.8) to 241 (±177), though statistical significance was not reached (P=0.07). With respect to NK function, a significant difference in the functional ability of NK cells to kill tumor target cells was found after TMD therapy. The percent lysis before and after therapy were 9.4% (±3.5) and 14.5% (± 4.4), respectively (P= 0.001), representing a 35% increase.
Discussion
The results presented here indicate that use of the thermomechanical massage device (TMD) enhances T and NK cell-mediated immune functions in healthy young adult subjects, and it may provide a complementary means to boosting the immune system.
The therapeutic effects of acupuncture on WBC proportion and function have been studied in healthy subjects and in patients with immune disorders and cancer. These include a decrease in lymphocyte values in healthy subjects (10), an increase in the relative percentage of total T-cell population (CD3+) and CD4+ T-lymphocyte subpopulation(29,30) and increased blastogenic response to PHA. Long-term massage therapy has been reported to cause an increase in the number of T cells and NK cells and their functions in both cancer patients and patients with HIV disease (22, 23 30, 32). An increase in NK proportion and function has also been reported by other devices such as acupuncture (30,37,38), moxibustion (29), and massage therapy (22, 23, 31,32, 39).
NK cells are a major component of the innate immune system that have the ability kill tumor cells (33, 34) and cells infected with viruses (35, 36). T lymphocytes are a major component of adaptive immune system and they play an important regulatory role in the generation and termination of the immune response. Our results showed that use of TMD caused a significant increase in the proportion of CD4+ and CD8+ T cells expressing CD25 and in the proportion NK cells. In this study, we found no increase in the absolute number of T cells and NK cells following thermal mechanical massage therapy, despite an increase in the proportions of these cells in peripheral blood. This may be due to a decrease, although not significant, in the absolute number of lymphocytes following thermal mechanical massage.
Two mechanisms can be proposed for the increase in T cell and NK cell function following thermal mechanical massage. First, it is possible that the observed increase in proportion of T lymphocytes and NK cells following TMD may, in part, account for the enhancement in T cell proliferation and increased NK cytotoxic function. However, it should be mentioned that T and NK cell functional activity is regulated by expression of activating and/or inhibitory receptors (40, 41). Therefore, a decrease or increase in cell number in PBMCs may not necessarily result in a decrease or increase in T and NK cell activity, but rather may be due to absence of negative signaling. In this context, monocytes/macrophages are shown to provide negative signaling to T cells and NK cells (42,42,43,44,45). The finding that the proportion of monocytes decreased following TMD supports the view that decreased negative signaling might have contributed to increased T and NK cell function. Alternatively, TMD might affect the state of activation of T and NK cells. In support of this hypothesis, we found that the proportion of T-cells expressing the CD25 cell surface marker (activated cells) changed as a result of thermal mechanical massage. The observed increase in the T cell and NK proprtions and decreased monocyte proportion may have been the result of catecholamines and neurotransmitters stimulating the release of lymphocytes into the circulation and sequestering monocytes.
The mechanism(s) by which TMD alters the proportion of different cell populations is not known, but could be mediated through the autonomic nervous system (ANS) and the central nervous system (CNS). Recent studies revealed that stimulation of acupuncture points either through massage, insertion of needles or heat induce activation of the ANS (16,17,18,19) In addition, other studies using new imaging modalities, such as functional MRI and PET scans, have revealed a direct effect of acupuncture points on the function of different brain centers(26,27). It is now well recognized that the immune system is modulated by the ANS (24,25) via a structural link between the ANS and the immune system. This link was uncovered through several studies that demonstrated ANS innervation of the lymphoid tissues (45,46,47,48) and observed unmyelinated nerves adjacent to lymphocytes in mammalian lymphoid tissues (49, 50). Numerous studies have also demonstrated that the immune system is modulated by the central nervous system (51, 52). In addition, neurotransmitters (including neuropeptides) may directly modulate lymphocyte differentiation, as is suggested by the presence of special receptors for most neurotransmitters on the surfaces of lymphocytes (24, 25,53). Further studies are needed to elucidate whether TMD activates the ANS and/ or CNS.
Conclusion
We conclude that TMD treatment may have immunostimulatory effects. Although the results are based on a small number of subjects, the findings are encouraging and additional studies are needed on a larger scale. These preliminary findings have potential importance in validating and expanding the therapeutic uses of massage, since they suggest that massage may have health benefits beyond and unrelated to its stress-reduction potential. They need to be replicated, however, because of the small sample size and lack of a control group. Additionally, it would be important to clearly distinguish cumulative effects from those from a single treatment and to demonstrate long-term effects, if any, on immunity and subsequent health status.
Acknowledgement
We wish to thank Migun Corporation, USA, for providing the Migun HY-7000 bed. We also want to thank to UCI students (Bio 199) Heung Joo Sung, and Yun Soo Kim for assisting us in data entry.
Correspondence to
Sastry Gollapudi, Ph.D., Department of Medicine, Room C240, Medical Sciences Building 1, University of California Irvine, CA 92697, U.S.A. Tel: (949) 824-6072, Fax: (949) 824-4362. E-mail address: svgollap@uci.edu
References
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 343:1-19, 2004.
2. Thomas K, Coleman P. Use of complementary or alternative medicine in a general population in Great Britain. Results from the National Omnibus survey. J Public Health (Oxf) 2004; 26:152-157.
3. Yamashita H, Tsukayama H, Sugishita C. Popularity of complementary and alternative medicine in Japan: a telephone survey. Complement Ther Med. 2002; 10:84-93.
4. Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. Jama. 1998; 280:1569-1575,
5. Astin J. Why patients use alternative medicine: results of a national study. Journal of the American Medical Association.1998; 279:1548-1553.
6. Druss BG and Rosenheck RA. Association between use of unconventional therapies and conventional medical services. Journal of the American Medical Association. 1999;282: 651-656.
7. So CS, Giolli RA, Chang T, Bae H-J, Chang Y, Boone WR and Blanks RH. Physiological Changes Following Thermomechanical Massage in a population of Hypertensive and/or Type II Diabetics.2004; J. Vertebral Subluxation Research.1-9..
8. Nordschow M, and Bierman W. The influence of manual massage on muscle relaxation: effect on trunk flexion. J Am Phys Ther Assoc1962;. 42:653-657.
9. Smith LL, Keating MN, Holbert D, Spratt DJ, McCammon MR, Smith SS and Israel RG. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: a preli. 1994.
10. Kou W, Bell JD, Gareus I, Pacheco-López G, Goebel MU, Spahn G, Stratmann M, Janssen OE, Schedlowski M and Dobos GJ,. Repeated acupuncture treatment affects leukocyte circulation in healthy young male subjects: A randomized single-blind two-period crossover study. Bain, Behavior, and Immunity. 2005; 19:318-324.
11. Hansen T and Kristensen JH. Effect of massage, shortwave diathermy and ultrasound on 133Xe disappearance rate from muscle and subcutaneous tissue in human calf. Scand J Rehabil Med.1973; 5:179-182.
12. Kurz W and Kurz R, Litmanovitch YI, Romanoff H, Pfeifer Y and Sulman FG. Effect of manual lymphdrainage massage on blood components and urinary neurohormones in chronic lymphedema. Angiology 1981;.;32:119-127.
13. Uchida S, Suzuki A, Kagitani F, Nakajima K and Aikawa Y. Effect of moxibustion stimulation ofvarious skin areas on cortical cerebral blood flow in anesthetized rats. Am J Chin Med 2003;;31:611-621.
14. Field T, Morrow C, Valdeon C, Larson S, Kuhn C, and Schanberg S. Massage reduces anxiety in child and adolescent psychiatric patients. J AM Acad Child Adolesc Psychaitry.1992; 31:125-131.
15. Cabyoglu MT, Ergene N and Tan U. The mechanism of acupuncture and clinical applications.2006; Int J Neurosci. February 116:115-125.
16. Moffet HH. How might acupuncture work?. A systematic review of physiologic rationales from clinical trials. BMC Complement Altern Med 2006; 6:25.
17. Cho ZH, Hwang SC, Wong EK, Son YD, Kang CK, Park TS, Bai SJ, Kim YB, Lee YB, Sung KK, Lee BH and Shepp LA, Min KT. Neural substrates, experimental evidences and functional hypothesis of acupuncture mechanisms.2006; Acta Neurol Scand. 113:370-377.
18. Tiran D and Chumman H. The physiological basis of reflexology and its use as a potential diagnostic tool. Complement Ther Clin Pract. 2005; 11:58-64.
19. Hadfield N. The role of aromatherapy massage in reducing anxiety in patients with malignant brain tumours. Int J Palliat Nurs 2001;;7:279-285.
20. Kim CK, Choi GS, Oh SD, Han JB, Kim SK, Ahn HJ, Bae H and Min BI. Electroacupuncture up-regulates natural killer cell activity Identification of genes altering their expressions in electroacupuncture induced up-regulation of natural killer cell activity. 2005;J Neuroimmunol. 168:144-153.
21. Wu B. [Effect of acupuncture on the regulation of cell-mediated immunity in the patients with malignant tumors].1995; Zhen Ci Yan Jiu. 20:67-71.
22. Birk TJ, McGrady A, MacArthur RD and Khuder S. The effects of massage therapy alone and in combination with other complementary therapies on immune system measures and quality of life in human immunodeficiency virus. J Altern Complement Med.6:405-414, 2000.
23. Shor-Posner G, Miguez MG, Hernandez-Reif M, Perez-Then E and Fletcher M. Massage treatment in HIV-1 infected Dominican children: a preliminary report on the efficacy of massage therapy to preserve the immune system in children without antiretroviral medication.2004; J Altern Complement Med. 10:1093-1095.
24. Kin NW and Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol 2006; 79:1093-1104.
25. Downing JE and Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today 2000;. 21:281-289.
26. Hui KK, Liu J and Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 2005;.27:479-496.
27. Cho ZH, Oleson TD, Alimi D and Niemtzow RC. Acupuncture: the search for biologic evidence with functional magnetic resonance imaging and positron emission tomography techniques. J Altern Complement Med2005;. 8:399-401.
28. So CS, Giolli RA, Jauregui M, Schuster TL, Yang H and Blanks R. Thermomechanical Massage Devices used in China and South Korea: A Preliminary Report of Health Outcomes and Side Effects. J Vertebral Subluxation Res 2003; 1-9.3.
29. Mori H, Nishijo K, Kawamura H and Abo T. Unique immunomodulation by electro-acupuncture in humans possibly via stimulation of the autonomic nervous system. Neurosci Lett. 2002;320:21-24.
30. Kung YY, Chen FP and Hwang SJ,. The Different Immunomodulation of Indirect Moxibustion on Normal Subjects and Patients with Systemic Lupus Erythematosus. The American Journal of Chinese Medicine. 2006;34:47-56.
31. Goodfellow LM. Effects of Therapeutic Back Massage on .Psychophysiologic Variables and Immune Function in Spouses of Patients with Cancer. Nursing Research2003;;52:318-328.
32. Iwama H and Akama Y. Skin rubdown with a dry towel activates natural killer cells in bedridden old patients. Med Sci Monit. 8:CR611-615, 2002.
33. Lotzova, E. Natural killer cells: immunobiology and clinical prospects.
34. 1991;9:173-84.
35. Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R and Moretta A. Human natural killer cells: their origin, receptors and function. Eur. J. Immunol. 2002; 32: 1205-1211.
36. Leong CC, Chapman TL, Bjorkman PJ, Formankova D, Mocarski ES, Phillips JH and Lanier LL Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I major histocompatibility complex and a viral class I homolog. J. Exp. Med.1998; 187: 1681-1687.
37. Biron CA, Nguyen KB, Pien GC, Cousens LP and Salazar-Mather TP Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17: 189-220.
38. Sato T, Yu Y, Guo SY, kasahara T and Hisamitsu T. Acupuncture stimulation enhances splenic natural killer cytotoxicity in Rats. Jpn J Physiol 1996.; 46:131-136.
39. Hahm ET, Lee JJ, Lee Wk, Bae HS, Min BI and Cho YW. Electoacupuncture enhancement of natural killer cell activity suppressed by anterior hypothalmic lesions in rats. Neuroimmunomodulation. 2004;11:268-272.
40. Hernadez-Reif M, Field T, Ironson G, Beutler J, Vera Y, Hurley J, Fletcher MA, Schanberg S, Kuhn C and Fraser M. Natural Killer cells and lymphocytes increase in woman with breast cancer following massage therapy. Int J Neurosci. 2005;115: 495-510.
41. Kroczek RA, Mages HW and Hutloff A. Emerging paradigms of T cell costimulation. Curr Opininons in Immunol 2004;16:321-327.
42. Leibson PJ. The regulation of lymphocyte activation by inhibitory receptors. Curr Opinions in Immunol. 2004; 16: 328-336.
43. Seaman WE, Gindhart TD Blackman MA Dala B Talal N and N Werb Z. suppression of natural killing in vitro by monocytes and polymorphonuclear leukocytes: requirement for reactive metabolites of oxygen\ J. Clin. Invest.1982; 69:876-878.
44. Ghoneum M and Abedi S, Enhancement of Natural Killer Cell Activity of Aged Mice by Modified Arabinoxylan Rice Bran (MGN-3/Biobran). J Pharm and Pharmacol, 2004; 56:1581-1588.
45. Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A and Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med.1999; 189: 1363-1372.
46. Bulloch, K. Neuroanatomy of lymphoid tissue: a review, in Guilemin R, Cohn M, Melnechuk T (eds): Neural Modulation of Immunity. New York, Raven Press, pp 55-73, 1985
47. Vulchanova L, Casey MA, Crabb GW, Kennedy WR and Brown DR.Anatomical evidence for enteric neuroimmune interactions in Peyer’s patches.2007; J Neuroimmunol. 185:64-74.
48. Moncrief K and Kaufman S. Splenic baroreceptors control splenic afferent nerve activity. Am J Physiol Regul Integr Comp Physiol. 2006; 290: R352-643.
49. Novotny GE, Schöttelndreier A and Heuer T.A light and electron microscopic quantitative analysis of the innervation of axillary lymph nodes in juvenile and old rats. J Anat. 1993,183:57-66.
50. Reilly FD, McCuskey RS and Meineke HA. Studies of the hemopoietic microenvironment. VIII. Adrenergic and cholinergic innervation of the murine spleen. Anat Res. 1976,185:109-17.
51. Calvo W. The innervations of the bone marrow in laboratory animals. Am J Anat. 1968;123:315-28.
52. Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA and Bellinger DL Bidirectional Communication between the Brain and the Immune System: Implications for Physiological Sleep and Disorders with Disrupted Sleep. Neuroimmunomodulation. 2006;13:357-74.
53. Darko DF. A brief tour of psychoneuroimmunology. Annals of Allergy. 1986; 57:233-238.
54. Ovadia H and Abramsky O. Dopamine receptors on isolated membranes of rat lymphocytes. J Neurosci Res. 1987;18:70-4






